Research
SphygmoCor® technology has been used in research and pharmaceutical trials worldwide in a broad range of therapeutic areas, including hypertension, renal disease, heart failure, inflammatory processes, diabetes, men’s health, women’s health, and COPD.
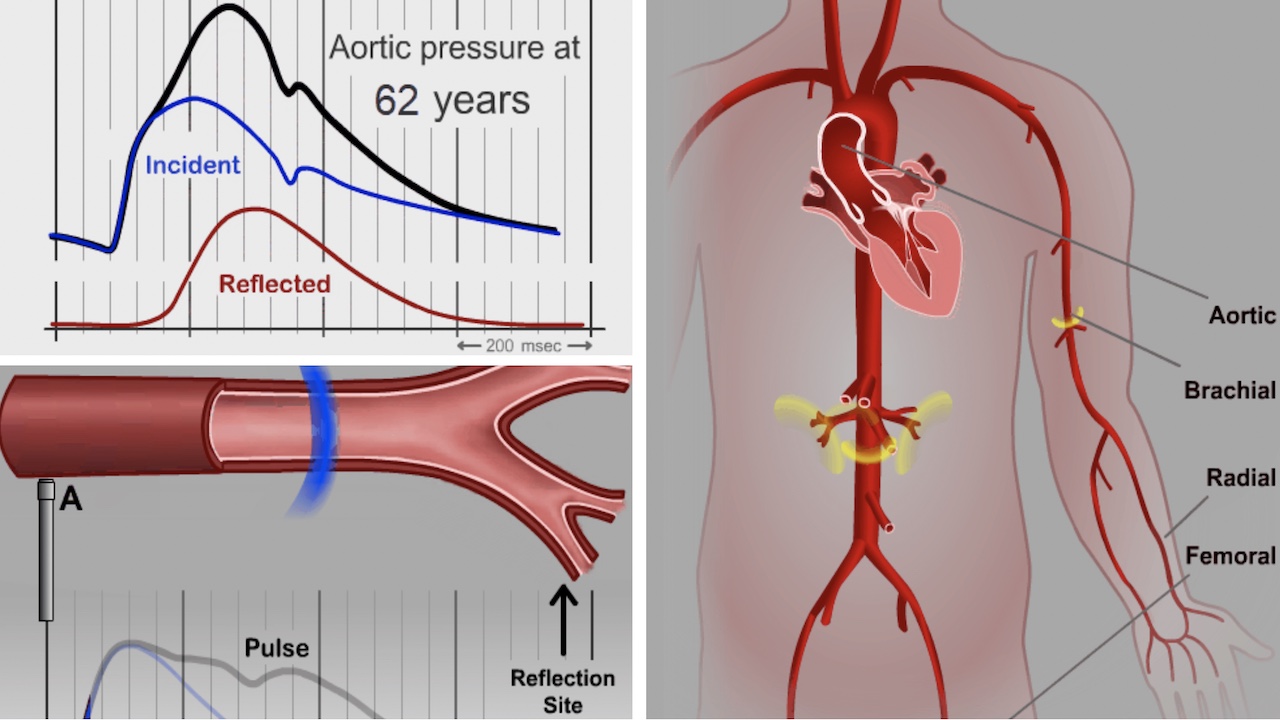
Major peer-reviewed studies, including the CAFE Study, Strong Heart Study, MESA Trial, Copenhagen Heart Study and others have established that patients with elevated central aortic blood pressure are at significantly higher risk for heart attack, stroke, heart failure, and kidney disease.

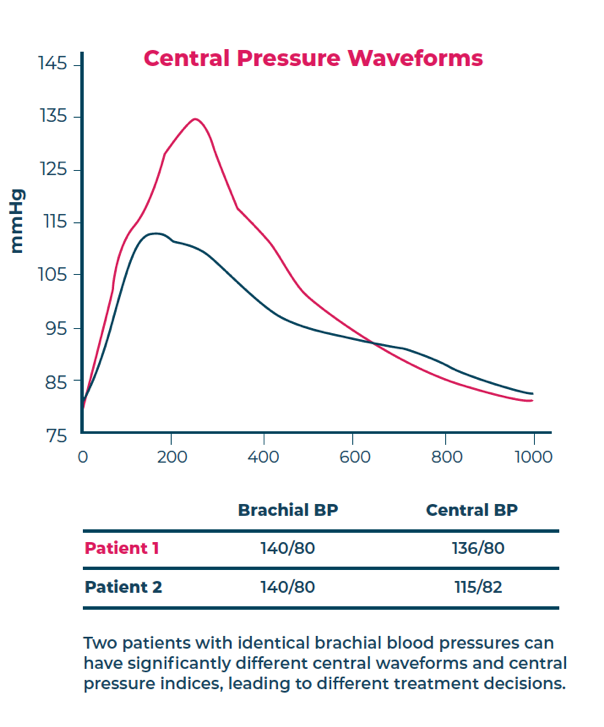
Further, numerous other outcomes studies have demonstrated significant individual variations between central aortic blood pressure and brachial blood pressure values—indicating that central aortic pressure and its related parameters cannot be inferred from brachial systolic and diastolic values. Pharmacological studies in all major classes of anti-hypertensive drugs have shown that the effects of drugs on central aortic pressure differs between and within classes of drugs, indicating that the effects of drug therapy on central arterial pressure waveforms cannot be inferred from standard brachial cuff blood pressure measurements.
Advanced Hemodynamic Assessment for Improved Insight During Clinical Trials
Central Measurements Offer Deeper Insights
Central hemodynamic assessments such as aortic pressure waveform analysis (PWA) and pulse wave velocity (PWV) measurement present offer a deeper understanding of the cardiovascular effects of an intervention. By characterizing ventricular-arterial interactions, pulse wave analysis can independently predict response to therapies, target organ damage, and risk of cardiovascular events. These important insights enhance investigators clinical program decision-making.
Comprehensive Cardiovascular Assessment
Standard cardiovascular evaluation tools traditionally used in clinical trials have limited ability to characterize the hemodynamic effects of pharmacological or device-based interventions.
ATCOR noninvasive central blood pressure (NcBP) waveform analysis and carotid-femoral PWV measurements directly assess aortic pressures and stiffness. These measurements bring value, confidence, and granularity to drug development and medical device interventions.Incorporating NcBP waveform analysis in your clinical trial allows you to:
- Assess potential off-target benefits of novel interventions
- Improve mechanistic understanding of medical interventions
- Inform future cardiovascular outcome trials
- Facilitate safety assessments
Clinical Trial Services
We provide a comprehensive suite of services for central hemodynamic data collection for clinical trials.
 ATCOR Technology
ATCOR Technology- Central arterial pressure waveform analysis (PWA)
- Carotid-femoral pulse wave velocity (PWV)
- Ambulatory blood pressure monitoring (ABPM)
 Training & Certification
Training & Certification- Study-specific training
- Operator certification
 Design & Development
Design & Development- Protocol design assistance
- Study-specific user manuals and training materials
 Technical Experience
Technical Experience- Dedicated team of technical experts
- 15 years’ experience
- Involvement in all phases of trial implementation
 Implementation
Implementation- Equipment logistics
- Simple, encrypted data transfer
- 24/7 site support
 Therapeutic Experience
Therapeutic Experience- Hypertension - COPD - Heart failure
- Diabetes - Chronic kidney disease
- Gout... and others
Worldwide Presence
Our expertise in cardiovascular hemodynamic data collection has been demonstrated in clinical trials throughout the world in all phases of development—from single-site trials to several hundred-site studies. Sponsors range from start-ups to top global pharmaceutical companies.
ATCOR Technology
SphygmoCor® is the industry standard platform for noninvasive central blood pressure (NcBP) and arterial stiffness assessment—vital data that deliver deep, individual clinical insights.
The SphygmoCor® XCEL System derives the central aortic pressure waveform using a standard blood pressure cuff. The procedure is easy to perform, reproducible, and can be seamlessly integrated into a clinical trial study visit.
The Oscar 2 with SphygmoCor® Inside ambulatory blood pressure monitor adds SphygmoCor® central aortic pressure waveform analysis to traditional ABPM.
The Clinical Trial Service Team provides expertise for a variety of technologies:
- Office pulse wave analysis (PWA) and brachial blood pressure (BBP)
- Carotid-femoral pulse wave velocity (PWV)
- 24-hour ambulatory brachial and central blood pressure and PWA
Pulse Wave Analysis (PWA)
ATCOR SphygmoCor® technology enables noninvasive measurement of the central aortic pressure waveform.
The predictive superiority of central blood pressure over brachial blood pressure is primarily due to the proximity of the ascending aorta to important target organs such as the heart, brain, and kidneys.1
Three aspects of central arterial PWA are especially important:
- Individual variability in the difference between central and brachial pressures can be significant and clinically important. 2-4
- Central pressures cannot be reliably inferred from brachial pressures. 2-4
- Medications may have significantly different effects on brachial blood pressure than on the central arterial pressure waveform. 5-7
PWA Data Elements
Key output parameters for the SphygmoCor® XCEL and Oscar 2 with SphygmoCor® Inside include:
- Aortic Augmentation Pressure
- Aortic Augmentation Index
- Aortic Systolic Blood Pressure
- Aortic Diastolic Blood Pressure
- Forward Wave Amplitude
- Reflected Wave Amplitude
- Reflection Magnitude
- Mean Arterial Pressure
Carotid Femoral Pulse Wave Velocity (PWV)
Carotid-femoral PWV is the AHA’s recommended method for noninvasive measurement of arterial stiffness.8 It is widely considered the most powerful cardiovascular risk factor and a valuable biomarker for cardiovascular risk prediction.
Elevated PWV (increased aortic stiffness) is a precursor to hypertension and its persistent elevation during treatment is associated with high risk for an adverse outcome in those with established disease.
Citations and References
References
- Hashimoto J. Tohoku J Exp Med 2014;233:1-8.
- O’Rourke et al. Br J Clin Pharmacol 2001;51:507-22.
- Sharman JE et al. J Hum Hypertens 2008; Dec 22(12):838-844.
- McEniery et al. Hypertension 2008; 6(51):1476-1482.
- Protogerou, AD et al. Curr Pharmaceut Des 2009; 15:272-289.
- McEniery, CM. Current Hypertension reports, 2009; 11:253-259.
- Townsend, RR et al. J Clin Hypertens 2015 Jul; 17(7):503-13.
- Townsend, RR et al. Hypertens 2015 Sep; 66(3); 698-722.