Diversity in FDA Clinical Trials Gets a Boost From New Legislation

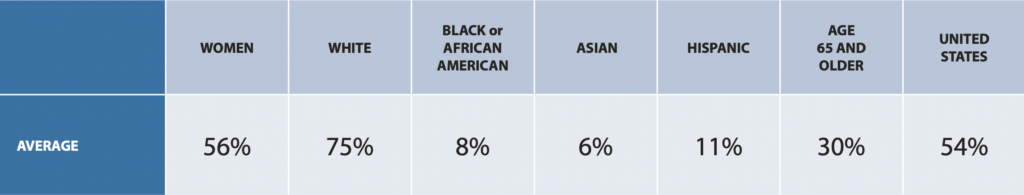
Clinical trials have historically fallen short of consistently and adequately representing diverse populations across therapeutic areas. On average, 75% of clinical trial participants are white, 8% are Black or African America, 6% are Asian, and 11% are Hispanic, according to a 2020 Drug Trials Snapshots Summary Report from the U.S. Food and Drug Administration.
To address this ongoing inequity, the FDA recently enacted the DEPICT Act – Diverse and Equitable Participation in Clinical Trials. The legislation, which mandates drug and device companies increase diversity, requires sponsors to submit a strategic diversity action plan timed with the study protocol submission detailing how phase three trials and other pivotal drug studies (other than bioavailability or bioequivalence studies) will expand trial enrollment to a broader population.1 By increasing diversity in clinical trials, the FDA hopes to more closely align drug and/or medical device safety and effectiveness data with race and/or ethnicity, closing knowledge gaps on how these products would work in large segments of the population that were previously excluded from trials.2,3
Participation in Clinical Trials in 2020
1Hyman, Phelps & McNamara, The FDA Law Blog, January 24, 2023: Under FDORA, FDA to Require Most Drug and Device Trials to Submit Diversity Action Plans (thefdalawblog.com).
2November 2020 FDA guidance: Enhancing the Diversity of Clinical Trial Populations – Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry (fda.gov)
3April 2022 FDA draft guidance: Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry (fda.gov)




